環(huán)境工程學報, 12(12): 3341-3350
楊瑞麗,王曉君,吳俊斌,等. 厭氧氨氧化工藝快速啟動策略及其微生物特性[J]. 環(huán)境工程學報,2018,12(12):3341-3350.
YANG Ruili, WANG Xiaojun, WU Junbin, et al. Rapid start-up strategy and microbial characteristics of anammox process[J]. Chinese Journal of Environmental Engineering,2018,12(12):3341-3350.







DOI 10.12030/j.cjee.201804096 中圖分類號 X703 文獻標識碼 A
楊瑞麗,王曉君,吳俊斌,等. 厭氧氨氧化工藝快速啟動策略及其微生物特性[J]. 環(huán)境工程學報,2018,12(12):3341-3350.
YANG Ruili, WANG Xiaojun, WU Junbin, et al. Rapid start-up strategy and microbial characteristics of anammox process[J]. Chinese Journal of Environmental Engineering,2018,12(12):3341-3350.
厭氧氨氧化工藝快速啟動策略及其微生物特性
楊 瑞麗 1,2, 王 曉君 1, 吳 俊斌 1, 郭 焱 1,2, 張 召基 1 , 陳 少華 1,*
1. 中國科學院城市環(huán)境研究所,城市污染物轉(zhuǎn)化重點實驗室,廈門 361021
2. 中國科學院大學,北京 100049
第一作者:楊瑞麗(1989—),女,博士研究生,研究方向:水污染控制技術(shù)。E-mail:rlyang@iue.ac.cn
*
通信作者,E-mail:shchen@iue.ac.cn
收稿日期: 2018-04-13; 錄用日期: 2018-10-08
基金項目: 福建省自然科學基金資助項目(2015J05115);中國科學院城市環(huán)境研究所青年前沿項目(IUEMS201404)摘 要
為探討種泥投加及氮負荷提升方式對厭氧氨氧化(anaerobic ammonia oxidation, anammox)工藝啟動中微生物豐度及群落結(jié)構(gòu)的影響,采取先普通活性污泥馴化后再接種anammox種泥的方式啟動anammox工藝。結(jié)果表明在活性遲滯階段投加anammox菌種可以快速啟動anammox工藝。通過縮短水力停留時間的方式增加氮負荷并可以避免直接提高進水氮濃度導致的基質(zhì)毒性抑制,有利于達到更高的總氮去除負荷。穩(wěn)定運行時反應器的氮去除負荷達0.51 kg·(m3·d)−1,anammox菌基因豐度為4.92×109 copies·g−1 (以VSS計),占細菌總數(shù)的2.70%。啟動階段,反應器內(nèi)微生物多樣性逐漸下降,檢測到浮霉菌門中4個anammox菌屬,以Candidatus Jettenia和Candidatus Kuenenia為主要anammox菌屬。在接種污泥處于活性遲滯階段時,結(jié)合提高進水氮濃度、縮短水力停留時間和投加anammox菌種的方式可以快速啟動anammox工藝。
Rapid start-up strategy and microbial characteristics of anammox process
YANG Ruili 1,2, WANG Xiaojun 1, WU Junbin 1, GUO Yan 1,2, ZHANG Zhaoji 1 , CHEN Shaohua 1,*
1. Key Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China
2. University of Chinese Academy of Sciences, Beijing 100049, China
第一作者:楊瑞麗(1989—),女,博士研究生,研究方向:水污染控制技術(shù)。E-mail:rlyang@iue.ac.cn
*
Corresponding author,E-mail:shchen@iue.ac.cn
Abstract
In order to investigate the effects of seed sludge inoculation and increase mode for the influent nitrogen load on the microbial abundance and community in anaerobic ammonia oxidation (anammox) reactor during a start-up period, the anammox process was initiated by inoculating acclimated activated sludge with anammox seed sludge. Results showed that adding anammox strain at a lag stage was beneficial to the rapid start-up of pilot-scale reactor. An improved nitrogen removal rate (NRR) was achieved through shortening hydraulic retention time as well as increasing the influent nitrogen load, which was an effective means to avoid the inhibition of matrix toxicity caused by increasing the influent TN concentration. In the steady running phase, the NRR of 0.51 kg·(m3·d)−1 was realized, and the gene abundance of anammox bacteria reached 4.92×109 copies·g−1(calculated by VSS), accounting for 2.70% of the total bacteria. In the start-up phase, the microbial diversity in the reactor gradually decreased, four anammox bacteria genus belonging to Planctomycetes were identified, and the dominant genus of functional bacteria were Candidatus Jettenia and Candidatus Kuenenia. The anammox process was successfully start-up with short term by inoculating anammox seed sludge, raising influent nitrogen and shorting hydraulic retention time at the lag stage of inoculation sludge.
厭氧氨氧化(anaerobic ammonia oxidation, anammox)工藝因脫氮效率高、污泥產(chǎn)量低、無需外加碳源等優(yōu)勢倍受歡迎,已被用于污泥消化液、味精廢水、造酒廢水和制藥廢水等的處理應用中[1-4]。但anammox菌具有生長速率慢、倍增時間長、環(huán)境敏感度高等缺陷,是anammox工藝實際應用的重要瓶頸之一[5-6]。為了探討anammox工藝快速啟動的方法,前人做了大量研究,如考察反應器類型[7-8]、填料類型[9]、接種污泥源[10-12]及操作參數(shù)[13]等的影響。其中,污泥源研究多是基于接種污泥中直接投加anammox種泥的啟動方式。唐崇儉等[10]通過接種硝化反硝化污泥、短程硝化污泥、厭氧絮體污泥和厭氧顆粒污泥并投加2%的anammox種泥,于255 d時,成功啟動了中試(2.5 m3)anammox反應器。WANG等[11]接種實驗室低溫保藏的anammox污泥,歷時160 d,成功啟動短程硝化厭氧氨氧化工藝。YE等[12]接種混合好氧、厭氧及同步部分硝化、厭氧氨氧化和反硝化污泥,于140 d時,氮去除負荷(nitrogen removal rate, NRR)達0.44 kg·(m3·d)−1。盡管將anammox污泥跟其他污泥混合后同時投加,增加了種泥中anammox菌的起始豐度,但隨著菌體自溶階段的運行,活性污泥解體必然會產(chǎn)生一定量的有機物而抑制anammox菌生長[2,12,14]。此時若首先投加普通活性污泥,待啟動過程的停滯階段再投加anammox種泥,可以有效避免有機物對其生長的抑制,且大大減弱了其他細菌跟anammox菌的競爭。另一方面,由于氨氮(NH4+-N)和亞硝氮(NO2−-N)對anammox菌都有毒害作用,如果單純通過提高進水NH4+-N和NO2−-N濃度來增加進水氮負荷(nitrogen loading rate, NLR),則可能導致基質(zhì)毒性抑制[2,15],而通過縮短水力停留時間(hydraulic retention time, HRT)則可避免,且該方式少有研究。
高通量測序技術(shù)可同時獲取上百萬條DNA,并將其準確歸類,是鑒定微生物的有效手段[16]。對anammox反應器進行功能菌定量分析和微生物群落結(jié)構(gòu)分析,可以從功能菌群豐度和群落結(jié)構(gòu)2個方面研究反應器啟動過程微生物群落演化,為判斷反應器成功啟動與否提供直接依據(jù)[16-17],并為反應器的穩(wěn)定運行和調(diào)控提供科學依據(jù)。本研究探索采取先普通活性污泥馴化后再接種anammox種泥的方式快速啟動anammox工藝,并采用先逐步提高進水總氮(total nitrogen, TN)濃度后縮短HRT的方法提高進水NLR。同時利用Illumina Miseq測序和實時定量聚合酶鏈式反應(polymerase chain reaction, PCR)方法,分析了啟動過程中污泥微生物群落變化,為深入認識anammox工藝的啟動進程提供依據(jù)。
1 材料和方法
1.1 實驗裝置
實驗采用升流式厭氧氨氧化反應器,實驗裝置示意圖及反應器照片見圖1。反應器有效容積100 L,頂部加蓋,由螺釘和膠條密封,并預留有排氣孔。距底部2 cm和頂部10 cm處分別設(shè)有進水口和出水口,中部和底部設(shè)有污泥采樣口。反應器內(nèi)部裝填鮑爾環(huán)填料。內(nèi)設(shè)回流裝置,強化反應器內(nèi)溶液混合,預防進水端反應物濃度過高,也起到防止進水口堵塞的作用。反應器進水pH經(jīng)0.1 mol·L−1鹽酸調(diào)節(jié)至7.2左右,外部包裹電加熱帶以保證內(nèi)部恒溫(35±2) °C,避光運行,維持anammox菌適宜的生長環(huán)境。

1.2 實驗條件和運行策略
實驗用水為人工配制的模擬廢水,具體組成見表1。其中,進水NH4+-N和NO2−-N濃度均為30~500 mg·L−1,NO3−-N濃度維持在100 mg·L−1以內(nèi)。另外,微量元素配方為ZnSO4·7H2O 0.43 g·L−1、CoCl2·6H2O 0.24 g·L−1、MnCl2·4H2O 0.99 g·L−1、CuSO4·5H2O 0.25 g·L−1、NaMoO4·2H2O 0.043 g·L−1、NiCl2·6H2O 0.20 g·L−1、KH2PO4 20 g·L−1、H3BO3 0.014 g·L−1,并加入EDTA 20 g·L−1以促進微量元素的溶解。采集進出水水樣經(jīng)0.45 μm濾膜過濾后,分別采用納氏試劑法、N-(1-萘基)乙二胺分光光度法和紫外分光光度法測定樣品中的NH4+-N、NO2−-N和NO3−-N [18]。pH采用便攜式pH計(FG2-FK, METTLER TOLEDO, USA)測定;污泥樣品MLSS、MLVSS均按標準方法[19]測定。

表1 模擬廢水成分
Table 1 Chemical composition of synthetic wastewater
Table 1 Chemical composition of synthetic wastewater
anammox工藝啟動過程主要分為4個階段,分別是Ⅰ菌體自溶、Ⅱ活性遲滯、Ⅲ活性提高和Ⅳ活性穩(wěn)定階段[7,20]。本研究采用2階段接種方式啟動anammox反應器。首先接種80 L取自廈門市某污水處理廠二沉池的活性污泥,使反應器中ρ(SS)=47.05 g·L−1,ρ(VSS)=13.19 g·L−1。等到污泥處于anammox活性遲滯階段時(第60 天),投加已培養(yǎng)10個月具有anammox活性的種泥(泥色微紅,ρ(SS)=26.69 g·L−1,ρ(VSS)=13.52 g·L−1),接種量為20 L。通過逐步提高進水TN濃度(41~116 d)和縮短HRT(117~172 d)來提高反應器NLR,具體運行參數(shù)見表2。

表2 反應器各階段運行參數(shù)
Table 2 Reactor operation parameters of each phase
Table 2 Reactor operation parameters of each phase
1.3 實驗裝置DNA提取、實時定量PCR分析與多樣性測定
1.3.1 DNA提取
分別在工藝啟動0、59、76、83、94、104、116、126、138、156和172 d時,采集污泥混合液。經(jīng)30 min靜沉后,稱取500 mg污泥樣品,使用FastDNA™ SPIN Kit for Soil(LLC, MP Biomedicals, USA)提取試劑盒,按其操作步驟提取污泥樣品中總DNA。DNA經(jīng)1%瓊脂糖凝膠電泳和Nanodrop(ND1000, Gene Company Limited, China)檢測其純度和濃度后進行實時定量PCR實驗,并從中選取0、59、104和172 d的DNA樣品進行Illumina高通量測序。
1.3.2 實時定量PCR分析
實時定量PCR是一種可以準確定量功能基因拷貝數(shù),進而計算功能菌豐度的實驗方法。本實驗采用Roche LightCycler® 480 Ⅱ(Roche Diagnostics Ltd., Rotkreuz, Swltzerland)實時熒光定量系統(tǒng)進行豐度分析,采用20 μL反應體系,具體配置為:SYBR Green Ⅰ Master(LightCycler® 480, mannheim, Germany)10 μL;前后引物各0.8 μL;質(zhì)粒或DNA樣品1 μL;去離子水 7.4 μL。其中,全細菌定量引物為通用引物341F:534R[21],而anammox菌采用特異性引物Amx808F(5'-ARC YGT AAA CGA TGG GCA CTA A-3')和Amx1040R (5'-CAG CCA TGC AAC ACC TGT RAT A-3')[21-22]。實時定量PCR運行程序為3步法:95 °C預變性5 min,35個循環(huán)(95 °C變性30 s,45 °C退火30 s,72 °C延伸30 s),72 °C終延伸10 min,最后進行溶解曲線分析,并計算anammox菌的絕對豐度及其在總細菌中的占比。
1.3.3 Illumina高通量測序
取樣品16S rRNA基因中的V4~V5區(qū),選用引物515F (5′-GTG CCA GCM GCC GCG G-3′)和907R (5′-CCG TCA ATT CMT TTR AGT TT-3′)進行擴增,擴增產(chǎn)物經(jīng)2%瓊脂糖凝膠電泳質(zhì)控,并均一化后,進行Miseq文庫構(gòu)建,并采用Illumina Miseq測序平臺對樣品進行高通量測序。源數(shù)據(jù)經(jīng)質(zhì)控處理后,篩選高質(zhì)量數(shù)據(jù)用MOTHUR軟件程序進行分析。
2 結(jié)果與討論
2.1 厭氧氨氧化工藝啟動過程脫氮性能變化
圖2為anammox反應器啟動過程各氮物質(zhì)濃度變化。反應器運行初期1~40 d為階段Ⅰ,出水NH4+-N和NO2−-N濃度均高于進水,為微生物的自溶解體階段[5,20]。該階段出水NO2−-N濃度逐漸下降,可能是反應器中共存有氨氧化菌(ammonia oxidizing bacteria, AOB)和反硝化菌(denitrification bacteria, DNB),其中AOB利用進水中微量的溶解氧將NO2−-N轉(zhuǎn)化為NO3−-N,DNB則將NO3−-N和NO2−-N還原為N2,使NO2−-N濃度下降[5,23]。CHAMCHOI等[7] 、GUO等[24]和BI等[25]研究表明,工藝啟動初期,反應器內(nèi)部微生物出現(xiàn)菌體細胞自溶,釋放出大量的有機物和NH4+-N,同時有機氮被分解,為異養(yǎng)微生物提供碳源,使出水NH4+-N和NO2−-N濃度高于進水,與本研究結(jié)果一致。反應器運行41~59 d為階段Ⅱ,此時出水NH4+-N和NO2−-N同步去除,表明anammox菌已顯示出一定活性[5,20,26]。ΔNO2−-N/ΔNH4+-N值平均為1.14,略低于STROUS等[27]報道的值,且出水NO3−-N濃度低于進水,說明反應器內(nèi)仍有一部分DNB利用殘留有機質(zhì)做電子供體還原NO3−-N為N2[28]。

圖2 中試厭氧氨氧化工藝啟動中進出水氮濃度變化
Fig. 2 Nitrogen variation of influent and effluent during start-up of anammox process in a pilot-scale reactor
Fig. 2 Nitrogen variation of influent and effluent during start-up of anammox process in a pilot-scale reactor
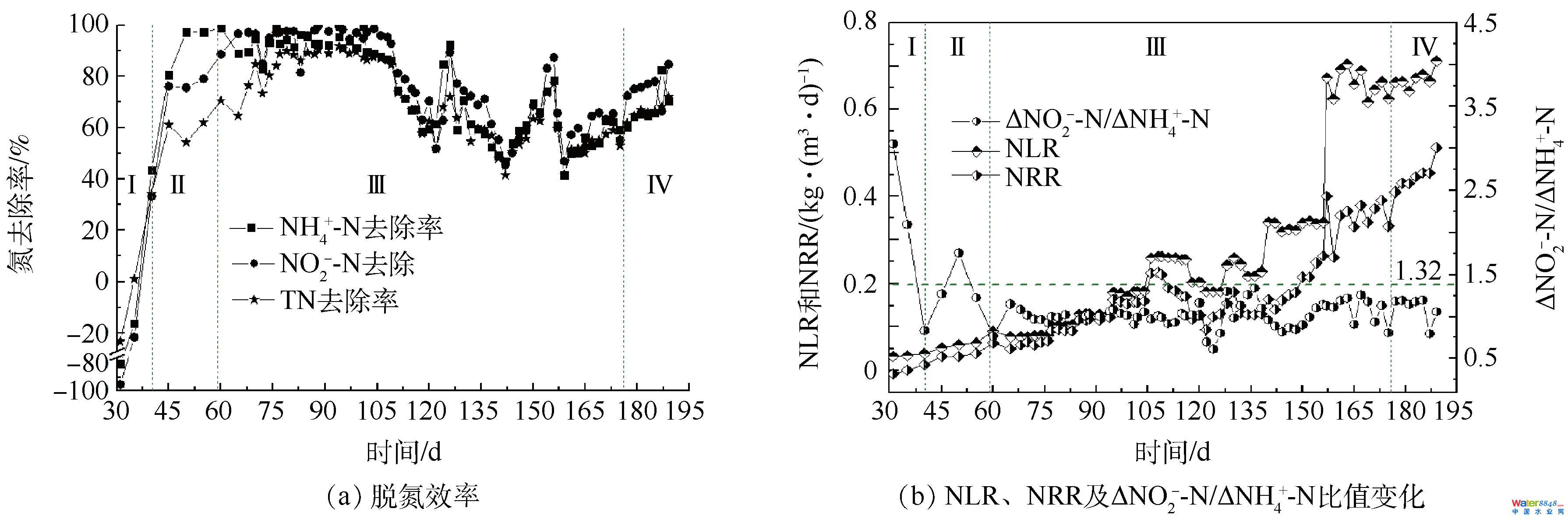
反應器運行第60 天,再接種20 L富含anammox菌的種泥,然后以提高進水氮濃度和縮短HRT的方式提高反應器NLR,此階段為階段Ⅲ。結(jié)合試厭氧氨氧化工藝啟動中進出水氮濃度變化(圖2)和厭氧氨氧化工藝啟動中脫氮效率、NLR、NRR及ΔNO2−-N/ΔNH4+-N比值的變化(圖3)可知,接種anammox種泥起到了穩(wěn)定反應器脫氮性能的作用,盡管在接種anammox種泥的同時,提高了進水TN濃度,也可使反應器于10 d內(nèi)TN去除率從64.70%提升至84.77%,促進了反應器的快速啟動[29]。這是因為經(jīng)過階段Ⅰ和Ⅱ的培養(yǎng)后,反應器內(nèi)可降解的有機碳源大幅降低,與anammox菌競爭的異養(yǎng)微生物顯著減少,此時添加有anammox活性的種泥可使反應器內(nèi)anammox菌豐度迅速增加,利于anammox活性的快速提升[30]。唐崇儉等[10]和WANG等[11]指出anammox種泥的添加是anammox工藝脫氮性能增強的關(guān)鍵。

圖3 厭氧氨氧化工藝啟動中脫氮效率、NLR、NRR及ΔNO2−-N/ΔNH4+-N比值變化
Fig. 3 Nitrogen removal performance of anammox reactor, including nitrogen removal efficiency, NLR, NRR and Δnitrite/Δammonium
Fig. 3 Nitrogen removal performance of anammox reactor, including nitrogen removal efficiency, NLR, NRR and Δnitrite/Δammonium
當進水TN濃度≤700 mg·L−1(60~104 d)時,雖然進水TN濃度持續(xù)增加,但反應器出水氮濃度并無明顯變化,反應器NRR達0.16 kg·(m3·d)−1。當進水TN濃度達1 000 mg·L−1(105~116 d)時,TN去除率于12 d內(nèi)由87.51%下降至66.89%,NRR較105 d時下降了24.16%。第116天,出水NH4+-N和NO2−-N濃度分別為104 d時的4.26和26.67倍,這可能是因為過高的NO2−-N濃度抑制了anammox菌的活性。曹雁等[17]也發(fā)現(xiàn),當進水NH4+-N和NO2−-N濃度分別達到330 mg·L−1和430 mg·L−1時,反應器出水水質(zhì)突然惡化。研究表明,NO2−-N對anammox菌的影響高于NH4+-N [31],NH4+-N濃度低于1 000 mg·L−1時不會抑制anammox菌活性,而NO2−-N高于280 mg·L−1時便會產(chǎn)生抑制[32],DAPENA-MORA等[33]也表明350 mg·L−1的NO2−-N會抑制anammox菌50%的活性。為確保反應器快速恢復并穩(wěn)定運行,在反應器運行117~126 d期間,將進水TN濃度降至700 mg·L−1,同時HRT由原來的4 d縮短為3.5 d,NLR也相應地由0.25 kg·(m3·d)−1降至0.19 kg·(m3·d)−1。由于進水基質(zhì)濃度降低對anammox菌的抑制作用解除,反應器anammox活性快速恢復,10 d內(nèi)NH4+-N、NO2−-N和TN去除率分別由67.27%、73.55%和66.89%快速升至92.41%、89.47%和72.05%。反應器運行127~172 d,HRT由3.5 d縮短至2 d,進而縮短至1 d,每次縮短HRT,反應器出水水質(zhì)都有一個惡化進而恢復的過程,表明功能菌群逐步適應著生長環(huán)境的變化[34]。第172天,NH4+-N、NO2−-N和TN去除率及NRR分別達60.82%、65.16%、57.70%和0.38 kg·(m3·d)−1。與提高氮濃度對反應器脫氮性能的影響相比,縮短HRT對提高反應器脫氮性能作用更大。研究表明,縮短HRT一方面會加速混合反應基質(zhì),另一方面會加大系統(tǒng)水流剪切力,這有利于anammox反應器的運行[35-36]。
階段Ⅳ(173~189 d)為anammox反應器的活性穩(wěn)定階段,最終NH4+-N、NO2−-N和TN去除率分別穩(wěn)定在76.50%、76.45%和70.04%左右,NRR達0.51 kg·(m3·d)−1。汪瑤琪等[34]在小試19.6 L的反應器中,將好氧污泥和anammox種泥以2:1的體積比混合啟動anammox工藝,耗時157 d,使NRR達0.44 kg·(m3·d)−1,本研究結(jié)果較其略有優(yōu)勢。JIN等[26]提出以NRR 達0.5 kg·(m3·d)−1為anammox反應器成功啟動的標準,表明中試anammox工藝已成功啟動。在反應器穩(wěn)定運行時,ΔNO2−-N:ΔNO3−-N:ΔNH4+-N平均為1.16:0.06:1,低于厭氧氨氧化反應化學計量式,說明仍有一定的DNB共存[26]。
2.2 厭氧氨氧化反應器啟動過程anammox菌豐度變化
2.2.1 污泥顏色變化
由圖4可知,初始接種時反應器內(nèi)污泥顏色為墨黑色。經(jīng)59 d厭氧馴化后,初顯anammox活性的污泥顏色轉(zhuǎn)為深灰色。隨著anammox活性提高污泥顏色逐步變紅,最終呈紅棕色。MOLINUEVO等[37]研究發(fā)現(xiàn),anammox菌因內(nèi)部含有大量血紅素C而呈現(xiàn)紅色,在本研究中,泥色逐漸變紅,進一步表明中試anammox工藝已成功啟動。

2.2.2 anammox菌豐度變化
由圖5可知,隨著anammox反應器運行時間延長,anammox菌基因豐度逐漸增加,最終豐度達4.92×109 copies·g−1 (以VSS計)。CHEN等[29]分別將反硝化和anammox種泥以3:1的體積比混合啟動anammox工藝,成功啟動時anammox菌基因豐度為4.13×109 copies·g−1。YIN等[9]采用添加氧化石墨烯的方式,促進anammox工藝的啟動,啟動成功時anammox菌基因豐度為1.84×109 copies·g−1。本研究結(jié)果高于二者成功啟動時的anammox菌基因豐度。16S rRNA基因豐度無顯著性變化,始終維持在(2.03±2)×1011 copies·g−1,anammox菌占16S rRNA的百分比由起初的0.01%漸增至2.70%。反應器運行1~59 d,anammox菌基因豐度由接種時的7.31×107 copies·g−1增至5.62×108 copies·g−1,增加了7.7倍。同時,16S rRNA基因豐度由5.20×1011copies·g−1下降至1.09×1011 copies·g−1,表明anammox菌開始逐步成為主要菌群之一[16]。經(jīng)過60~172 d的活性提高,anammox菌占16S rRNA的百分比由之前的0.51%增加至2.70%。CHEN等[29]接種常規(guī)反硝化污泥啟動anammox反應器,最終anammox菌占16S rRNA的百分比為3%左右。HU等[38]以接種水稻土啟動SBR型anammox反應器,18個月后,anammox菌占16S rRNA的百分比約為5.3%。對比文獻研究結(jié)果,從anammox菌絕對豐度和相對豐度兩方面均表明anammox反應器已成功啟動,且用時較HU等[38]縮短了6個月。

圖5 厭氧氨氧化工藝啟動中anammox菌基因豐度變化
Fig. 5 Changes in gene abundance of anammox bacteria during start-up of anammox process
Fig. 5 Changes in gene abundance of anammox bacteria during start-up of anammox process
2.3 厭氧氨氧化反應器快速啟動中菌群多樣性的變化
2.3.1 厭氧氨氧化反應器啟動中Alpha多樣性分析
厭氧氨氧化反應器啟動過程中Alpha多樣性指數(shù)的變化見表3。從表3可以看出,厭氧氨氧化反應器啟動中高通量測序的4個樣品覆蓋率指數(shù)均高于0.99,表明檢測結(jié)果基本涵蓋所有微生物的16S rRNA基因[34]。表3中觀察物種、Chao1和ACE指數(shù)表示樣品中物種數(shù)量的多少,值越大,物種越豐富;Shannon、Simpson和PD whole tree指數(shù)表示微生物群落組成的復雜度,其值越高,復雜度越高[39-40]。隨反應器NLR的提高,6種多樣性指數(shù)總體呈下降的趨勢,表明隨著馴化時間的延長,反應器內(nèi)微生物豐富度及復雜度降低。

表3 厭氧氨氧化反應器啟動過程中Alpha多樣性指數(shù)變化
Table 3 Alpha diversity analysis during start-up of anammox process in a pilot-scale reactor
Table 3 Alpha diversity analysis during start-up of anammox process in a pilot-scale reactor
2.3.2 門水平物種豐度分析
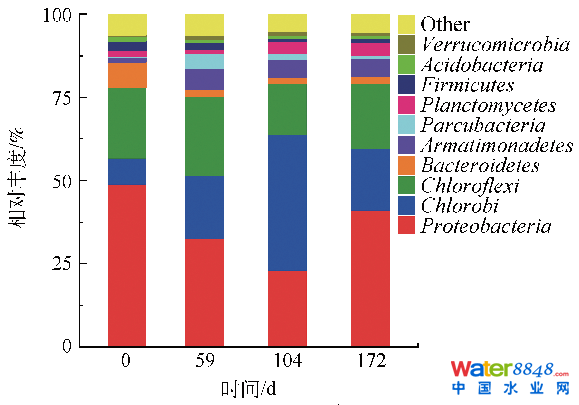
由圖6可知,反應器運行期間相對豐度較高的菌群主要有變形菌門(Proteobacteria)、綠菌門(Chlorobi)、綠彎菌門(Chloroflexi)、擬桿菌門(Bacteroidetes)、裝甲菌門(Armatimonadetes)、Parcubacteria門、浮霉菌門(Planctomycetes)等。無論是在廢水處理、厭氧消化還是土壤中,變形菌門均為最常見的貫穿始終的優(yōu)勢菌群[38,41-42],其豐度可在10.3%~55.5%間波動,為主要的脫氮功能菌群之一。本研究中,變形菌門相對豐度隨反應器NLR的提升,呈先降后升的趨勢。反應器運行0~104 d,由馴化初期的48.87%逐漸下降至22.92%,后于172 d時回升至41.02%,與CONNAN等[43]在anammox馴化過程中報道的變形菌門的變化趨勢相同。綠彎菌門、擬桿菌門和裝甲菌門是常見的可與anammox菌共存的菌群[44],同Parcubacteria門一起,其相對豐度均隨馴化而先降低后上升,變化范圍分別為15.48%~23.97%、1.80%~7.56%、1.47%~6.65%和0.26%~4.46%。CAO 等[16]對啟動成功的anammox-UASB反應器內(nèi)的污泥樣品進行了群落結(jié)構(gòu)分析,發(fā)現(xiàn)變形菌門、綠彎菌門、擬桿菌門、硝化螺旋菌門、酸桿菌門、裝甲菌門、綠菌門和浮霉菌門為主要菌門,變形菌門和綠彎菌門占據(jù)比值分別為27.07%~32.24%和25.77%~29.14%,并指出綠彎菌門和擬桿菌門與污泥顆粒的形成相關(guān)。CONNAN等[43]研究報道,anammox反應器內(nèi)微生物菌門主要為變形菌門、擬桿菌門、硬壁菌門、綠彎菌門、綠菌門、酸桿菌門和浮霉菌門,變形菌門豐度高達55.9%,綠彎菌門和綠菌門是anammox反應器內(nèi)常見菌門。
浮霉菌門為主要的自養(yǎng)脫氮功能菌群,現(xiàn)在已知的具有厭氧氨氧化功能的微生物均屬于浮霉菌門[45]。其變化趨勢與變形菌門相反,隨反應器NLR的提升,浮霉菌門相對豐度由0 d時的1.34%逐漸升高至172 d的3.64%,表明anammox菌得到了一定的富集。綠菌門相對豐度的變化趨勢與浮霉菌門變化趨勢一致。本研究結(jié)果與CAO 等[16]和CONNAN等[43]的結(jié)果相近,表明anammox顆粒污泥的形成可能受益于綠彎菌門、擬桿菌門、裝甲菌門、綠菌門和Parcubacteria門豐度的增加。

圖6 中試anammox反應器啟動過程中主要菌群變化(門水平)
Fig. 6 Variation of main microbial flora at a phylum level during start-up of anammox process in a pilot-scale reactor
Fig. 6 Variation of main microbial flora at a phylum level during start-up of anammox process in a pilot-scale reactor
2.3.3 浮霉菌門中屬水平物種豐度分析
為進一步了解anammox反應器啟動中內(nèi)部anammox菌的變化,選取浮霉菌門進行屬水平的物種豐度分析。浮霉菌門主要有I-8、Candidatus Jettenia、Candidatus Kuenenia、SM1A02和Planctomyces等。在已知的具有厭氧氨氧化能力的6個屬[45]中,本中試反應器檢測到4個,分別是Candidatus Jettenia、Candidatus Kuenenia、Candidatus Brocadia和Candidatus Anammoximicrobium。
由表4可知,中試anammox反應器內(nèi)Candidatus Jettenia和Candidatus Kuenenia為anammox的主要菌屬。同時,兩者也是污水處理廠和大規(guī)模anammox反應器中常見的2個屬[46] 。之后隨反應器內(nèi)NLR逐漸增大,Candidatus Jettenia和Brocadia屬豐度呈先增加后下降的趨勢,反應器運行59~104 d,分別由13.50%和1.50%逐漸增至39.51%和1.72%,后于172 d時降至5.26%和0.20%。而Candidatus Kuenenia屬豐度隨NLR增大而逐漸增加,第172天時Kuenenia屬豐度為第59 天時豐度的3.33倍,而Candidatus Anammoximicrobium屬豐度隨NLR增大而逐漸降低,第172 天時已檢測不到,可能是因為Candidatus Kuenenia的生長速率、亞硝酸鹽親和力及對水質(zhì)變化的抵抗力比其他anammox菌屬強[47-48]。運行期間,anammox菌占總細菌的百分比逐漸增加,最高可達1.65%(Jettenia+Kuenenia+Brocadia+Anammoximicrobium)。實時定量PCR結(jié)果顯示,在172 d時,anammox反應器中anammox菌的相對豐度為2.7%,與Illumina高通量測序結(jié)果接近,進一步印證了anammox工藝已快速啟動。

表4 浮霉菌門中部分菌屬的相對豐度變化
Table 4 Changes in relative abundance of some genus of planctomycetes
Table 4 Changes in relative abundance of some genus of planctomycetes
3 結(jié)論
1)在接種污泥處于anammox活性遲滯階段時,投加富含anammox菌的種泥可以快速啟動anammox反應器,且在anammox活性提高階段,通過縮短HRT能夠避免直接提高進水TN濃度帶來的基質(zhì)毒性抑制,可以更大程度上提高反應器氮去除負荷,啟動成功后,anammox反應器最終NRR達0.51 kg·(m3·d)−1。
2)在啟動過程中,接種污泥顏色由墨黑色逐漸變?yōu)榧t棕色,anammox菌豐度隨NLR的增加逐漸上升,最終基因豐度達4.92×109copies·g−1 (以VSS計),占總細菌的2.70%。
3)在工藝啟動階段,anammox反應器內(nèi)微生物復雜度逐漸降低,于浮霉菌門中檢測到4個anammox菌屬:Candidatus Jettenia、Candidatus Kuenenia、Candidatus Brocadia和Candidatus Anammoximicrobium屬,占測序總數(shù)的1.65%,Candidatus Jettenia和Candidatus Kuenenia是反應器中主要的anammox菌屬。
參考文獻
- WR V D S, ABMA W R, BLOMMERS D, et al. Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale anammox reactor in Rotterdam[J]. Water Research,2007,41(18):4149-4163. [CrossRef]
- TANG C, PING Z, YI Y U, et al. Monosodium glutamate wastewater treatment with a full-scale SHARON reactor[J]. Acta Scientiae Circumstantiae,2008,28(11):2228-2235.
- DENG L W, ZHENG P, CHEN Z A. Anaerobic digestion and post-treatment of swine wastewater using IC-SBR process with bypass of raw wastewater[J]. Process Biochemistry,2006,41(4):965-969. [CrossRef]
- TANG C J, ZHENG P, CHEN T T, et al. Enhanced nitrogen removal from pharmaceutical wastewater using SBA-ANAMMOX process[J]. Water Research,2011,45(1):201-210. [CrossRef]
- LU Y F, MA L J, MA L, et al. Improvement of start-up and nitrogen removal of the anammox process in reactors inoculated with conventional activated sludge using biofilm carrier materials[J]. Environmental Technology,2017,39(1):59-67. [CrossRef]
- IBRAHIM M, YUSOF N, MOHD Z M Y, et al. Enrichment of anaerobic ammonium oxidation (anammox) bacteria for short start-up of the anammox process: A review[J]. Desalination & Water Treatment,2016,57(30):13958-13978. [CrossRef]
- CHAMCHOI N, NITISORAVUT S. Anammox enrichment from different conventional sludges[J]. Chemosphere,2007,66(11):2225-2232. [CrossRef]
- TAO Y, GAO D W, FU Y, et al. Impact of reactor configuration on anammox process start-up: MBR versus SBR[J]. Bioresource Technology,2012,104(1):73-80. [CrossRef]
- YIN X, QIAO S, ZHOU J, et al. Fast start-up of the anammox process with addition of reduced graphene oxides[J]. Chemical Engineering Journal,2016,283:160-166. [CrossRef]
- 唐崇儉, 鄭平, 陳建偉,等. 中試厭氧氨氧化反應器的啟動與調(diào)控[J]. 生物工程學報,2009,25(3):406-412.
- WANG G, XU X, ZHOU L, et al. A pilot-scale study on the start-up of partial nitrification-anammox process for anaerobic sludge digester liquor treatment[J]. Bioresource Technology,2017,241:181-189. [CrossRef]
- YE L, LI D, ZHANG J, et al. Fast start-up of anammox process with mixed activated sludge and settling option[J]. Environmental Technology,2017,19:1-8. [CrossRef]
- WANG T, ZHANG H, YANG F, et al. Start-up and long-term operation of the anammox process in a fixed bed reactor (FBR) filled with novel non-woven ring carriers[J]. Chemosphere, 2013,91(5):669-675. [CrossRef]
- WETT B. Solved upscaling problems for implementing deammonification of rejection water[J]. Water Science & Technology,2006,53(12):121-128. [CrossRef]
- WANG Y, MA X, ZHOU S, et al. Expression of the nirS, hzsA, and hdh genes in response to nitrite shock and recovery in candidatus kuenenia stuttgartiensis[J]. Environmental Science & Technology,2016,50(13):6940-6947. [CrossRef]
- CAO S, DU R, LI B, et al. High-throughput profiling of microbial community structures in an ANAMMOX-UASB reactor treating high-strength wastewater[J]. Applied Microbiology & Biotechnology,2016,100(14):6457-6467. [CrossRef]
- 曹雁, 王桐嶼, 秦玉潔,等. 厭氧氨氧化反應器脫氮性能及細菌群落多樣性分析[J]. 環(huán)境科學,2017,38(4):1544-1550. [CrossRef]
- HENDRICKX T L, KAMPMAN C, ZEEMAN G, et al. High specific activity for anammox bacteria enriched from activated sludge at 10°C[J]. Bioresource Technology,2014,163:214-221. [CrossRef]
- 國家環(huán)境保護總局. 水和廢水監(jiān)測分析方法[M].4版. 北京: 中國環(huán)境科學出版社,2002:211-213.
- TANG C J, ZHENG P, CHAI L Y, et al. Characterization and quantification of anammox start-up in UASB reactors seeded with conventional activated sludge[J]. International Biodeterioration & Biodegradation,2013,82(8):141-148. [CrossRef]
- HOU L, ZHENG Y, LIU M, et al. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary[J]. Journal of Geophysical Research Biogeosciences,2014,118(3):1237-1246. [CrossRef]
- LI M, HONG Y, KLOTZ M G, et al. A comparison of primer sets for detecting 16S rRNA and hydrazine oxidoreductase genes of anaerobic ammonium-oxidizing bacteria in marine sediments[J]. Applied Microbiology & Biotechnology,2010,86(2):781-790. [CrossRef]
- BAGCHI S, BISWAS R, ROYCHOUDHURY K, et al. Stable partial nitrification in an up-flow fixed-bed bioreactor under an oxygen-limiting environment[J]. Environmental Engineering Science,2009,26(8):1309-1318. [CrossRef]
- GUO J, WANG S, LIAN J, et al. Rapid start-up of the anammox process: Effects of five different sludge extracellular polymeric substances on the activity of anammox bacteria[J]. Bioresource Technology,2016,220:641-646. [CrossRef]
- BI Z, QIAO S, ZHOU J, et al. Fast start-up of anammox process with appropriate ferrous iron concentration[J]. Bioresource Technology,2014,170(5):506-512. [CrossRef]
- JIN R C, ZHENG P, HU A H, et al. Performance comparison of two anammox reactors: SBR and UBF[J]. Chemical Engineering Journal,2008,138(1):224-230. [CrossRef]
- STROUS M, HEIJNEN J J, KUENEN J G, et al. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms[J]. Applied Microbiology & Biotechnology,1998,50(5):589-596. [CrossRef]
- WANG S, GUO J, LIAN J, et al. Rapid start-up of the anammox process by denitrifying granular sludge and the mechanism of the anammox electron transport chain[J]. Biochemical Engineering Journal,2016,115:101-107. [CrossRef]
- CHEN H, HU H Y, CHEN Q Q, et al. Successful start-up of the anammox process: Influence of the seeding strategy on performance and granule properties[J]. Bioresource Technology,2016,211:594-602. [CrossRef]
- 陳光輝, 李軍, 鄧海亮,等. 包埋菌啟動厭氧氨氧化反應器及其動力學性能[J]. 化工學報,2015,66(4):1459-1466. [CrossRef]
- PUYOL D, CARVAJALARROYO J M, SIERRAALVAREZ R, et al. Nitrite (not free nitrous acid) is the main inhibitor of the anammox process at common pH conditions.[J]. Biotechnology Letters,2014,36(3):547-551.
- ISAKA K, SUMINO T, TSUNEDA S. High nitrogen removal performance at moderately low temperature utilizing anaerobic ammonium oxidation reactions[J]. Journal of Bioscience & Bioengineering,2007,103(5):486-490. [CrossRef]
- DAPENA-MORA A, CAMPOS I F L. Evaluation of activity and inhibition effects on anammox process by batch tests based on the nitrogen gas production[J]. Enzyme & Microbial Technology,2007,40(4):859-865. [CrossRef]
- 汪瑤琪,張敏,姜瀅,等.厭氧氨氧化啟動過程及微生物群落結(jié)構(gòu)特征[J]. 環(huán)境科學,2017,38(12):5184-5191.
- 孫佳晶. 厭氧氨氧化菌的富集培養(yǎng)及乙酸影響研究[D]. 遼寧:大連海洋大學, 2014.
- 秦永麗, 蔣永榮, 劉成良,等. Anammox啟動過程中脫氮性能及污泥特性研究[J]. 環(huán)境科學與技術(shù),2016,39(2):122-127.
- MOLINUEVO B, GARCIA M, KARAKASHEV D, et al. Anammox for ammonia removal from pig manure effluents: effect of organic matter content on process performance[J]. Bioresource Technology,2009,100(7):2171-2175. [CrossRef]
- HU M, WANG X, WEN X, et al. Microbial community structures in different wastewater treatment plants as revealed by 454-pyrosequencing analysis[J]. Bioresource Technology,2012,117(10):72-79. [CrossRef]
- SCHLOSS P D, GEVERS D, WESTCOTT S L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies[J]. Plos One,2011,6(12):e27310. [CrossRef]
- WANG Y, BU C N, KANG Q, et al. Autoclaved sludge as the ideal seed to culture anammox bacteria: Reactor performance and microbial community diversity[J]. Bioresource Technology,2017,244:391-399. [CrossRef]
- RIVIÉRE D, DESVIGNES V, PELLETIER E, et al. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge[J]. ISME Journal,2009,3(6):700-714. [CrossRef]
- FIERER N, BRADFORD M A, JACKSON R B. Toward an ecological classification of soil bacteria[J]. Ecology,2007,88(6):1354-1364. [CrossRef]
- CONNAN R, DABERT P, KHALIL H, et al. Batch enrichment of anammox bacteria and study of the underlying microbial community dynamics[J]. Chemical Engineering Journal,2016,297:217-228. [CrossRef]
- KINDAICHI T, YURI S, OZAKI N, et al. Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor[J]. Water Science & Technology,2012,66(12):2556-2561. [CrossRef]
- ALI M, OKABE S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues[J]. Chemosphere,2015,141:144-153. [CrossRef]
- KUENEN J G. Anammox bacteria: from discovery to application[J]. Nature Reviews Microbiology,2008,6(4):320-326. [CrossRef]
- WR V D S, MICLEA A I, VAN DONGEN U G, et al. The membrane bioreactor: A novel tool to grow anammox bacteria as free cells[J]. Biotechnology & Bioengineering,2008,101(2):286-294. [CrossRef]
- ISAKA K, SUWA Y, KIMURA Y, et al. Anaerobic ammonium oxidation (anammox) irreversibly inhibited by methanol[J]. Applied Microbiology & Biotechnology,2008,81(2):379-385. [CrossRef]